Innovation
Game Teaches Coping Skills to Kids Exposed to Trauma

“Mwa-ha-ha-haaaa!” laughs General Malaise, a character in a game-like, online tool under development called “Coping Coach” designed to be played by school-age children who have experienced a traumatic event. The web-based intervention is based on areas that researchers at The Children’s Hospital of Philadelphia and others have shown to be important in lowering the severity of pediatric post-traumatic stress.
These include teaching children to recognize helpful or unhelpful thoughts and behaviors and how not to rely on avoidance as a coping response. For example, General Malaise goes on to zap the town, leaving the townspeople without any feelings, and then the player must identify different emotions in order to advance to the next module.
“Each module includes carefully selected intervention targets based in empirical evidence on how post-traumatic stress develops in children,” said Meghan Marsac, PhD, a CHOP psychologist at the Center for Injury Research and Prevention (CIRP) and research assistant professor at the Perelman School of Medicine at the University of Pennsylvania, who has co-led the development and evaluation of Coping Coach. “We have applied what we know about the treatment of post-traumatic stress to prevention.”
A study team tested Coping Coach’s feasibility in a randomized controlled trial of 72 children ages 8 to 12 who had been admitted to the hospital for an acute medical event. They invited one group of children to log in and play the game within six weeks after being admitted to the hospital. A second group was assigned to a wait list and given the same instructions to complete the online activities at 12 weeks.
Both groups completed research assessments over the phone at six, 12, and 18 weeks so that the researchers could track their symptoms and coping skills over time. They concluded that both groups benefited from Coping Coach participation, which suggests its recommended timing can be flexible. The investigators reported their results in the Journal of Pediatric Psychology.
The next step is to test Coping Coach in a bigger trial, said Nancy Kassam-Adams, PhD, a CHOP psychologist and associate director of Behavioral Research at CIRP, who was the lead author of the study. Once the researchers have enough data to validate Coping Coach’s effectiveness, she anticipates that it could be publicly available within the next five years. Since the number of school-age children who could benefit from a low-cost, web-based post-traumatic stress intervention is enormous, she envisions Coping Coach as a way to fill the gap in resources available to support them during their recovery.
“It’s a different way of engaging kids,” Dr. Kassam-Adams said. “We’ve worked hard to build in items that are useful and therapeutic while keeping it fun. It doesn’t substitute for full-blown mental health treatment. This is for the early days after they’ve been through something difficult, and it teaches kids skills to recover well.”
Researchers Expand Evidence for Reprogrammed T Cell Therapy

Since the news broke of the first pediatric patient receiving an investigational immune cell therapy at The Children’s Hospital of Philadelphia in 2012, for leukemia that had relapsed twice after conventional treatments, the researchers continue to learn more from each clinical study participant who undergoes CTL019 treatment.
The investigational approach developed by a team at CHOP and the University of Pennsylvania is a personalized cell therapy that reprograms a patient’s own immune system to fight specific types of cancer cells. At the heart of the therapy are bioengineered T cells called CTL019 cells that potentially seek and destroy B-cells, including tumor cells, that express the antigen CD19, a protein essential to cell processes that appears on the cells’ surface.
“Our results show that these engineered cells greatly expand in patients, producing complete response rates over 90 percent,” said study leader Stephan A. Grupp, MD, PhD, a pediatric oncologist at CHOP and professor of Pediatrics at the Perelman School of Medicine at the University of Pennsylvania. “Just as important, the cells are persisting in patients, potentially allowing for long-term disease control.”
The Food and Drug Administration designated the CTL019 approach as a Breakthrough Therapy in July 2014. It is the first personalized cellular therapy under development for the treatment of cancer to receive this important classification, which helps to expedite its progress into broader clinical trials.
Multi-site Phase II clinical trials in pediatric acute lymphoblastic leukemia (ALL) are already underway to test this experimental cell therapy in more patients in several hospitals around the world, including CHOP. The trials are sponsored by Novartis Pharmaceuticals, which acquired exclusive rights to CTL019 from Penn in 2012.
Most recently, at the annual meeting of the American Society of Hematology in December 2015, the CHOP/Penn team reported that 55 of 59 children (93 percent) who received the treatment at CHOP experienced a complete remission. They reported that 18 patients had continuous remissions for more than one year, and nine patients for more than two years. At one year, 79 percent of these children with highly resistant ALL were still surviving.
“As we follow these patients for longer periods, we are seeing some patients stay in remission without further therapy,” said Shannon Maude, MD, PhD, a pediatric oncologist at CHOP and an assistant professor of Pediatrics at Penn. “We are continuing to learn about this therapy, and from this, hope to continue to improve.”
Most (88 percent) of the patients developed cytokine-release syndrome, a systemic inflammatory response ranging from mild forms, which may include fever and muscle aches, to severe forms, which may be life-threatening. A subset of patients experienced neurologic toxic effects ranging from delirium accompanying high temperature to global encephalopathy with additional symptoms, with these effects lasting over two to three days and fully resolved in all patients.
The CHOP study team had reported similar results in October 2014 in the New England Journal of Medicine.
“The patients who participated in these trials had relapsed as many as four times, including 60 percent whose cancers came back even after stem cell transplants,” Dr. Grupp said. “The durable responses we have observed with CTL019 therapy are unprecedented.”
Financial support for cell therapy research is helping to get new studies on line, including a $100,000 gift over the winter from Curing Kids Cancer to support cell therapy directed at an alternative target (CD22) in ALL, as well as a grant from Solving Kids Cancer to support cell therapy treatments for neuroblastoma.
“Support like this allows us to test these new treatments in kids, who are often at the back of the line for new cancer therapies,” Dr. Grupp said. “Given that drug companies usually target the adult market, it’s amazing that Novartis is planning to go for FDA approval in pediatric ALL.”
Ongoing research is vital because many questions remain unanswered, and the treatment is not yet proven.
“It’s so important to understand that, while we know so much more than we did starting out three years ago, this is still a limited group of patients,” Dr. Grupp said, adding that it is not yet known whether CTL019 represents a long-term solution or cure for the disease.
Rare Gene Discovery Platform a Perfect Match for Researchers
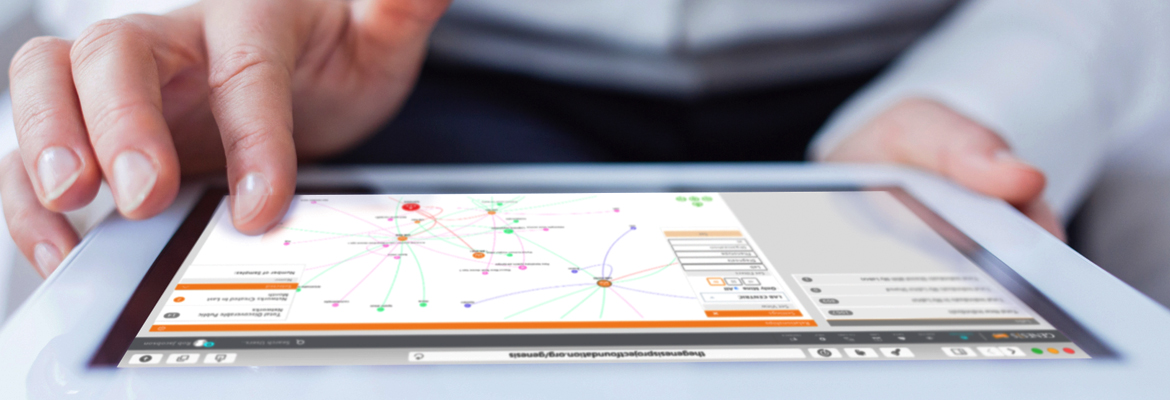
Discovery is in the air. New matchmaking approaches to explore genomic data are an alluring way for physician scientists to find connections, start conversations, and launch beautiful breakthroughs.
A curious researcher contemplating a seemingly unsolvable patient case can reach out to another investigator on the opposite side of the world studying a patient with a similar genetic makeup. Working together, in a matter of minutes they can pinpoint the genetic mutations that may explain the rare disease that their patients have in common.
“It is an important, new way of thinking about collaborative science and reaching beyond your own research studies to deepen your understanding,” said Marni Falk, MD, an attending physician and director of the Mitochondrial-Genetic Disease Clinic at The Children’s Hospital of Philadelphia.
Dr. Falk helped form the Mitochondrial Disease Sequence Data Resource (MSeqDR), a secure web-based portal that pools genomic information and is integrated with a real-time data analysis engine called GENESIS. The technologies’ user-driven data sharing capabilities enable scientists anywhere to gain novel insights into genes, variants, and phenotypes.
A researcher can submit a patient’s sequence data information — from a solitary gene to an entire genome — and then they can query it within the systems’ intuitive tools and shared databases, Dr. Falk explained. It lets investigators directly ask clinically meaningful questions: Show me mutations that are new in this person. Show me ones that they got from their mother. Show me ones that are in this particular biological pathway.
“The interface is so straightforward that it’s like clicking on Google as opposed to typing computer code,” Dr. Falk said. “Everything is provided in an interactive format so that the investigator can analyze their patient’s data by herself.”
Next-generation sequencing has evolved so rapidly that researchers have an unprecedented and vast amount of genomic information to comb through. For example, scientists who study mitochondrial diseases have identified more than 1,000 genes that are involved with making proteins that work in the mitochondria. Plus, mitochondria have their own DNA that also harbors disease-causing mutations. Subsequently, hundreds of genetic causes already have been implicated in mitochondrial diseases.
“It is a very computationally heavy field,” Dr. Falk said. “Most of the clinical world and the research world do not have the bioinformatics capabilities to facilely manipulate, explore, and share the data necessary to make new discoveries.”
That is why robust genomic data resources that are secure, user-friendly, quick, and economical — MSeqDR and GENESIS are free to academic users — are essential for accurate sequence variant annotation, analysis, sharing, and interpretation. Dr. Falk has led seminars across the globe, including in Japan, Spain, Finland, and Italy, to train clinicians and researchers alike on how to use these tools to curate and analyze genomic data without needing to rely on a bioinformatician. She was the organizer and co-leader of MSeqDR’s development since it began as a grassroots effort in 2012 facilitated by the United Mitochondrial Disease Foundation, the North American Mitochondrial Disease Consortium, and the National Institutes of Health.
As the MSeqDR project gained momentum in the mitochondrial disease research community, Dr. Falk and colleagues began working in 2013 with the creators of GENESIS, including Stephan Zuchner, MD, PhD, chair of Genetics at the University of Miami Miller School of Medicine, to expand and integrate the collaborative analysis tools. Originally called GEM.app, GENESIS’ initial purpose was to interrogate genomic sequencing data related to neurologic disease. Since many patients with suspected mitochondrial disorders have neurological manifestations, the two initiatives meshed handsomely.
In articles published in the journals Molecular Genetics and Metabolism and Human Mutation, Dr. Falk and her co-authors describe the projects and how their efforts have grown beyond mitochondrial and neurological diseases. GENESIS has about 600 registered users from 44 countries. So far they have revealed more than 70 novel gene disorders.
Even if a patient’s mutation is found to be in a gene already known to cause disease, tools like this enable researchers to prove a correct diagnosis and better understand the disease scope or range of severity. For clinicians, directly probing their patients’ genomic data over time could help to determine the best drug to prescribe a patient if he has a genetic variance that could slow down or speed up his drug metabolism.
“This type of resource takes advantage of vast number of whole-exome and whole-genome datasets that are being generated to harness their power for gene discovery, better understanding of the disease, and better understanding and treatment of the patient,” Dr. Falk said.
After meeting with a patient one afternoon, Dr. Falk logged into MSeqDR and GENESIS to trace a genetic variant that seemed to be running through the family. Genetic sequencing had suggested that her patient had two variants of unknown significance within one gene. She suspected these variants were the cause of an inherited disease, but she could not be certain because genetic samples from the patients’ parents were not available.
Dr. Falk looked in the databases and found two other people who each had these same two variants. She contacted the investigator who had input their data, and was speaking to him by telephone the next day. It turned out the cases were a father and daughter who each were affected with an undiagnosed medical condition that was identical to that of Dr. Falk’s patient.
The other researcher was not aware that the patients had variants in this gene and was extremely grateful to know this information. Now, he had a diagnosis for that family, and Dr. Falk knew that she probably had in hand the right diagnosis for her patient and did not have to look any further.
“All the time now, we’re solving complex cases,” Dr. Falk said. “New gene disorders are being discovered literally every day. It is awesome. There has never before been anything like this resource for scientific investigators.”
Graphene Microelectrodes Give Dynamic Look at Seizures
One of the most common disorders of the nervous system, epilepsy affects 2.7 million Americans of all ages, races, and ethnic backgrounds. An epileptic seizure takes place when spontaneous high-frequency bursting of neural networks temporarily interrupts normal electrical brain function. Pinpointing those neurons’ locations and plotting the intensity of their activity in real time has been difficult for researchers.
An electroencephalogram (EEG), which is a recording of brain activity, traditionally requires metal electrodes that cause interference when used in conjunction with sophisticated, multicellular calcium imaging techniques that investigators couple with high-speed microscopes to see and record when neurons are firing. Neuroscience researcher Hajime Takano, PhD, who works in Douglas Coulter, PhD’s epilepsy research laboratory at The Children’s Hospital of Philadelphia, collaborated with researchers from the University of Pennsylvania’s School of Engineering to test a new type of transparent, flexible microelectrode that they developed to solve this problem.
“The idea of applying this technology to basic neuroscience for brain recording is something new and very exciting,” said Dr. Takano, who also has an engineering background and is a research assistant professor in the Neurology Department at the Perelman School of Medicine at the University of Pennsylvania.
It is made of the strongest material known to man: graphene, a two-dimensional form of carbon only one atom thick. Because it is see-through, the graphene microelectrode allows for simultaneous optical imaging and electrophysiological recordings of neural circuits that can provide valuable information on individual cells, while at the same time probing the regions that they may span.
In a study published in Nature Communications, Dr. Takano; senior author Brian Litt, PhD; Penn Engineering postdoc Duygu Kuzum, PhD; and colleagues described how they were able to use the graphene microelectrode technology in combination with calcium imaging involving confocal and two-photon microscopy to observe seizure-like activity that they induced in neural tissue from rats. The investigators were able to obtain both high spatial and temporal resolution, which is the ability to discriminate between two points in space and time.
“By monitoring a seizure with the transparent electrodes and imaging individual neurons at the same time, we can try to pinpoint where a seizure started,” Dr. Takano said. “If there are repeated seizures, we can see if the seizure-initiating cell is always the same or not. And if there is an initiating cell, what is different about it?”
Development of the transparent microelectrode technology involved a multidisciplinary effort from Penn’s new Center for NeuroEngineering and Therapeutics, Penn’s departments of Neuroscience, Pediatrics, and Materials Science, and the Division of Neurology at CHOP.
Electronic Portal to Communicate Goals in ADHD Care

How important are communication methods for the families of children being treated for attention-deficit/hyperactivity disorder (ADHD)? Investigators at The Children’s Hospital of Philadelphia intend to find out.
Parents of children with ADHD are key decision makers regarding their treatment plans, but families may not know how to express their preferences and goals to physicians. Researchers at CHOP received a $2.1 million award from the Patient-Centered Outcomes Research Institute (PCORI) to test how an electronic portal could be used to facilitate family participation in shared clinical decision-making.
ADHD occurs in 5 to 8 percent of school-age children, and they often have difficulties at school and home due to a short attention span, impulsivity, and/or hyperactivity. Stimulant or non-stimulant medication and behavior management training are ADHD treatments that parents often seem reluctant to discuss with physicians or to communicate any concerns about safety and efficacy.
Alexander G. Fiks, MD, MSCE, a co-investigator on the PCORI project, has been developing the electronic ADHD portal based on a questionnaire called the ADHD Preferences and Goals Instrument created by CHOP’s PolicyLab. The patient engagement tool is intended to be used in a primary care setting to help doctors and families work together to choose effective, culturally sensitive treatment plans for ADHD that are acceptable to each family and result in improved treatment outcomes. Dr. Fiks is a PolicyLab faculty member, an urban primary care pediatrician at CHOP, and an assistant professor of Pediatrics at the Perelman School of Medicine at the University of Pennsylvania.
James Guevara, MD, MPH, an attending physician at CHOP, will lead the study, which will be conducted in 15 primary care facilities across Children’s Hospital’s Care Network. The study team plans to assess the effectiveness of the ADHD portal versus the ADHD portal in combination with a care manager in communicating patients’ and families’ treatment goals. The researchers expect to enroll roughly 300 children between the ages of 5 to 12 years, who will be randomized to one of the two groups. Parents will complete ADHD outcome measures at zero, three, six, and nine months.
Dr. Guevara and colleagues also plan on using feedback to improve the study. They will ask various stakeholders — including clinicians, parents, and teachers of children with ADHD — to advise the research team on study questions, the investigation’s design, and how the results are disseminated.
“Findings from this study will inform the use of communication strategies to share family preferences and goals among parents, teachers, and clinicians of children with ADHD,” said Dr. Guevara, who also is an associate professor of Pediatrics and Epidemiology at Penn, and a founding member of PolicyLab.
Established by 2010’s Affordable Care Act, PCORI funds comparative effectiveness research, with an eye toward improving “the quality and relevance of evidence available to help patients, caregivers, clinicians, employers, insurers, and policy makers make informed health decisions,” according to the PCORI website.
List of Patents

United States Patent No. 8,846,632
Linda B. Couto
Nucleic Acids for Targeting Multiple Regions of the HCV Genome
Compositions and methods effective for modulating Hepatitis C viral infection are provided.
United States Patent No. 8,816,054
Valder Arruda; Rodney Camire; Nicholas Iacobelli
Compositions and Methods for Enhancing Coagulation Factor VIII Function
Factor VIII variants and methods of use thereof are disclosed.
United States Patent No. 8,889,742
Peter Gruber
Use of HDAC and/or DNMT Inhibitors for Treatment of Ischemic Injury
The present invention provides methods of ameliorating or reducing the extent of ischemic injury, reperfusion injury, and myocardial infarction, by administering an inhibitor of histone deacetylase enzyme (HDAC) or an inhibitor of DNA methyltransferase enzyme (DNMT).
United States Patent No. 8,999,322
Sriram Krishnaswamy
Compositions and Methods for Modulating Hemostasis
Novel thrombin/prothrombin protease/zymogen variants which have anticoagulation activity and methods of use thereof are disclosed.
United States Patent No. 8,852,879
Harry Ischiropoulos
Materials and Methods for the Detection of Nitrated Fibrinogen
Compositions are disclosed for detecting a patient's risk for coronary artery disease. The compositions can determine the presence of nitrated fibrinogen which is linked with coronary artery disease. Kits for the detection of coronary artery disease are also provided.



